
The food industry has undergone some significant changes to enhance food safety and protect consumers. The U.S. Food and Drug Administration (FDA) has been at the forefront of this transformation, driving regulatory standards to ensure a safer and more secure food supply chain.
FSMA 204 Explained
The Food Safety Modernization Act (FSMA) is crucial legislation that was enacted in 2011 to prevent food-borne illnesses. The 204 Traceability Rule, accompanied by the Food Traceability List (FTL), outlines the requirements for food facilities to implement effective systems for tracking and tracing food products included within the list specifically. FSMA 204 applies to all facilities that are legally required to register with the FDA, including both domestic and foreign facilities that manufacture, process, pack or hold food for human or animal consumption within the United States.
The Food Traceability List was created using a risk ranking model, where each food product available in the market has been scored on its hazard potential. This risk ranking model takes into account various factors such as outbreak frequency and criticality. Within the list, you can find detailed information about each food product, which is crucial information in identifying and containing any potential outbreak of illness.
Traceability Requirements Under FSMA 204
FSMA 204 introduced a comprehensive set of regulations accompanied by the Food Traceability List, emphasizing preventative controls, risk-based approaches, and increased accountability across the entire food supply chain. Having identified the products included within the FTL, it is required that additional records are kept on these items specifically.
The FDA has defined a set of metrics called Key Data Elements (KDEs) that correspond with different events in the supply chain known as Critical Tracking Events (CTEs). These tracking events include:
- Harvesting
- Cooling
- Initial Packaging
- First Land-Based Receiver (Seafood)
- Shipping
- Receiving
- Transformation
The Key Data Elements (KDEs) which must be tracked throughout the CTEs consist of:
– Information about the food: Type of food, brand name, and any lot codes or other identifying information.
– Information about the immediate previous source of the food: The name, address, and phone number of the source, as well as any other relevant information about the source.
– Information about the immediate subsequent recipient of the food: The name, address, and phone number of the recipient, as well as any other relevant information about the recipient.
– Information about the movement of the food: The date the food was received and the date it was shipped to the next recipient.
Rule 204 officially took effect in January 2022, 60 days after it was published in the Federal Register. However, revisions confirmed an additional three-year period, making the final compliance deadline, January 20th, 2026. The extended timeline provides organizations with enough time to properly implement and adhere to the necessary compliance processes outlined in Section 204.
The Impact on FDA Audits
FDA audits have been significantly reshaped by FSMA 204, introducing heightened traceability requirements that make it mandatory for food companies to maintain meticulous traceability records throughout the entire supply chain. During FDA audits, inspectors now place a stronger emphasis on the organization’s comprehensive understanding of the supply networks – from sourcing raw materials to the distribution of finished food products.
The challenge lies in the need for robust traceability systems that can withstand rigorous scrutiny. By leveraging advanced tracking systems, food organizations can swiftly identify and trace the origin of specific food items, facilitating rapid response in the event of contamination or safety concerns.
AuditComply for FSMA 204 Compliance
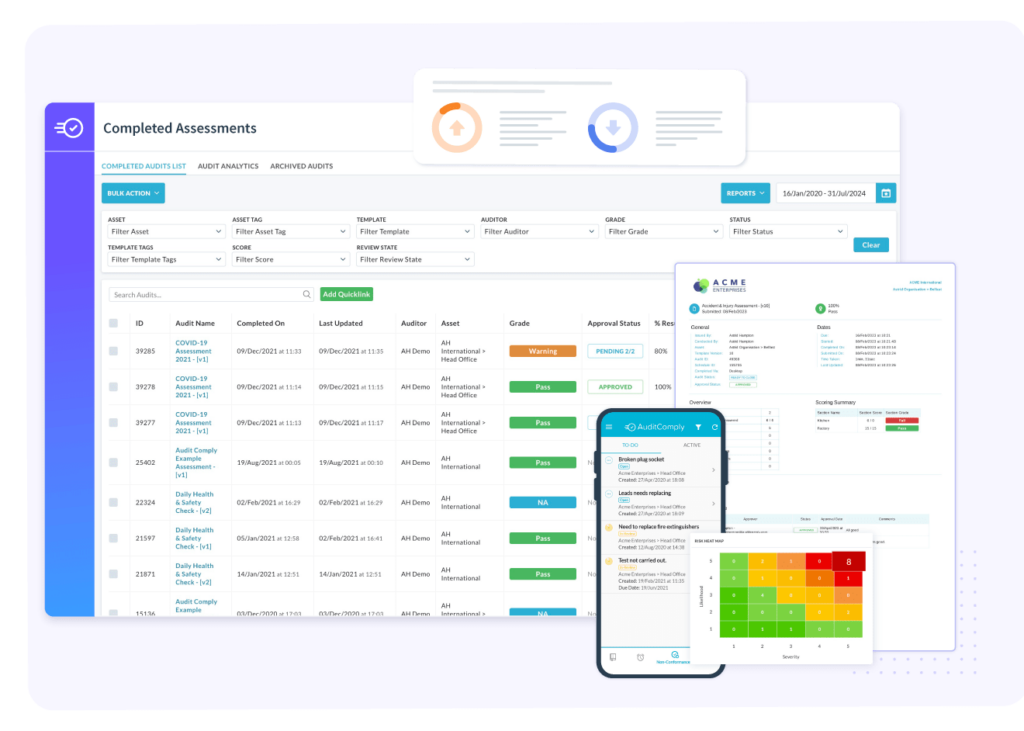
1. Enhanced Traceability Features: AuditComply offers robust traceability features that enable tracking of food products throughout the supply chain. Aligning with FSMA 204’s focus on improving traceability to address food safety issues quickly.
2. Digital Record-Keeping: FSMA 204 requires detailed record-keeping for high-risk foods. AuditComply’s digital record-keeping system ensures that all necessary documentation is maintained, organized and easily accessible, meeting this requirement.
3. Critical Tracking (CTEs) Management: AFSMA 204 mandates recording data at specific points in the supply chain, known as CTEs. AuditComply can be configured to capture and manage data at each CTE, ensuring compliance.
4. Real-Time Data Capture & Accessibility: The platform’s capability to capture and provide real-time data is crucial for the rapid response required by FSMA 204 in the event of food safety issues.
5. Supply Chain Transparency: AuditComply enhances supply chain transparency, providing a dedicated supplier environment where supplier collaboration is encouraged. Providing detailed insights into each stage of the food supply chain.
6. Risk Assessment Tools: AuditComply provides risk assessment and management, allowing real-time identification, management and control of food safety risks. The Risk Hub is connected to live events, identifying risks as they happen.
7. Audit & Inspection Readiness: AuditComply prepares customers for audits and inspections, which are integral to FSMA 204 compliance, by ensuring that all necessary data and documentation are in order and easily reviewable.

AuditComply is a revolutionary solution that empowers organizations to enhance their traceability management. Through its centralized repository of data, automated reporting and documentation features, and the ability to monitor production and distribution processes in real-time, we assist companies in safeguarding product safety and quality, adhering to legal and regulatory standards, and optimizing supply chain management.
Whether you are already an AuditComply customer or interested in discovering how AuditComply can help with your FSMA 204 compliance requirements, we encourage you to reach out to one of our experts today.
